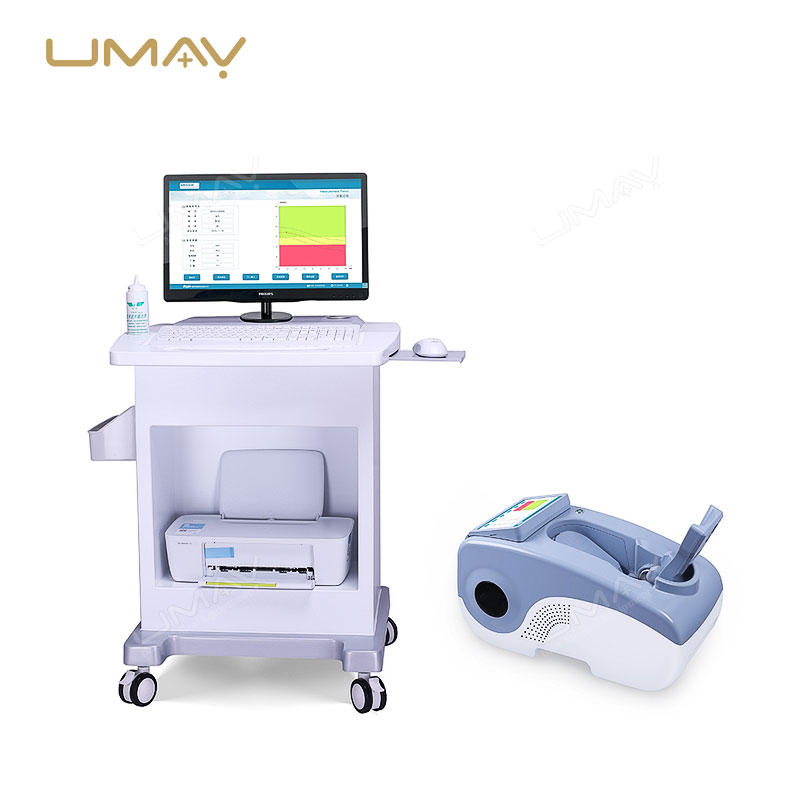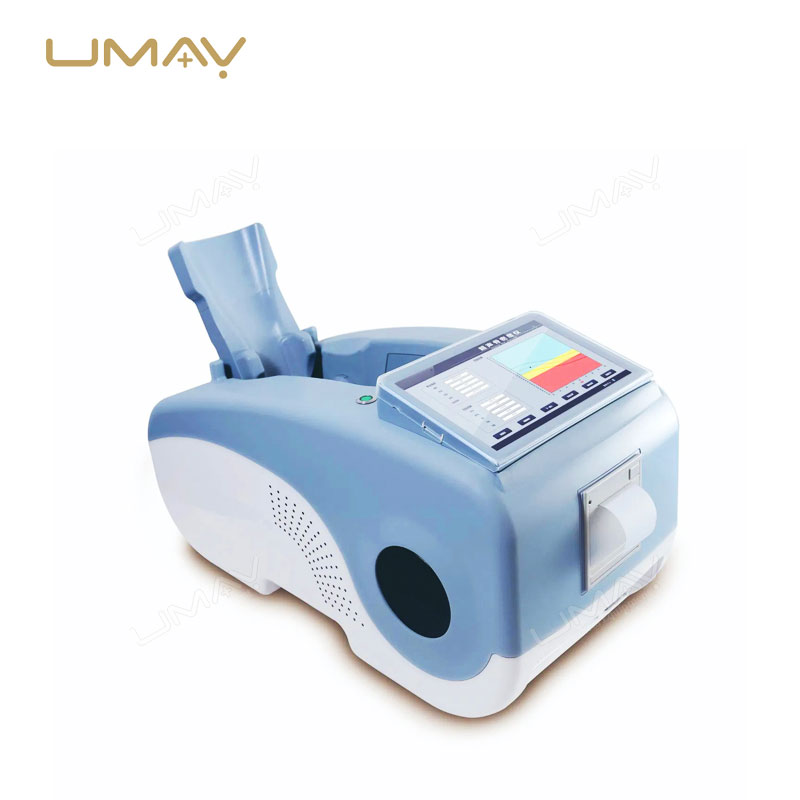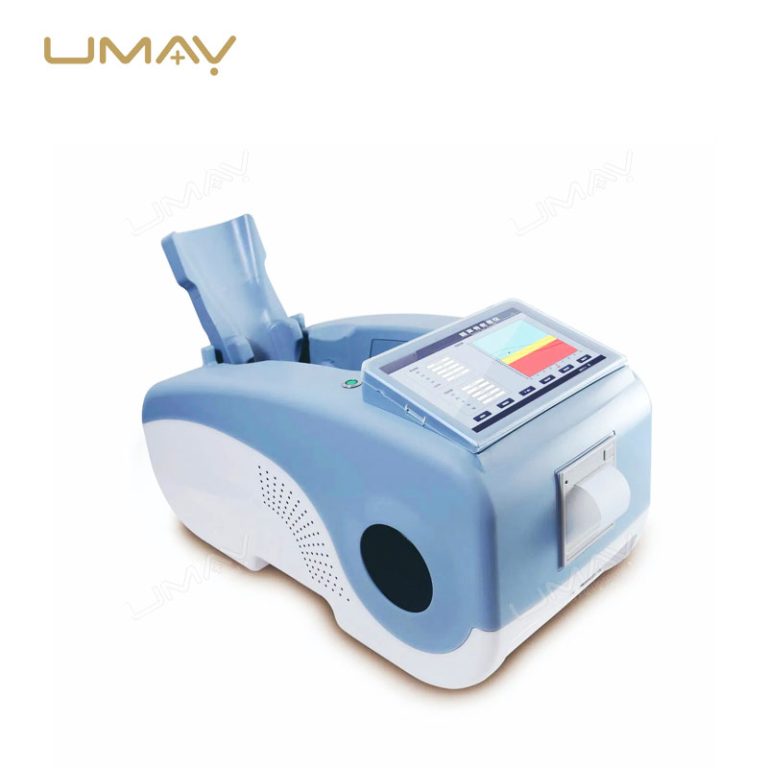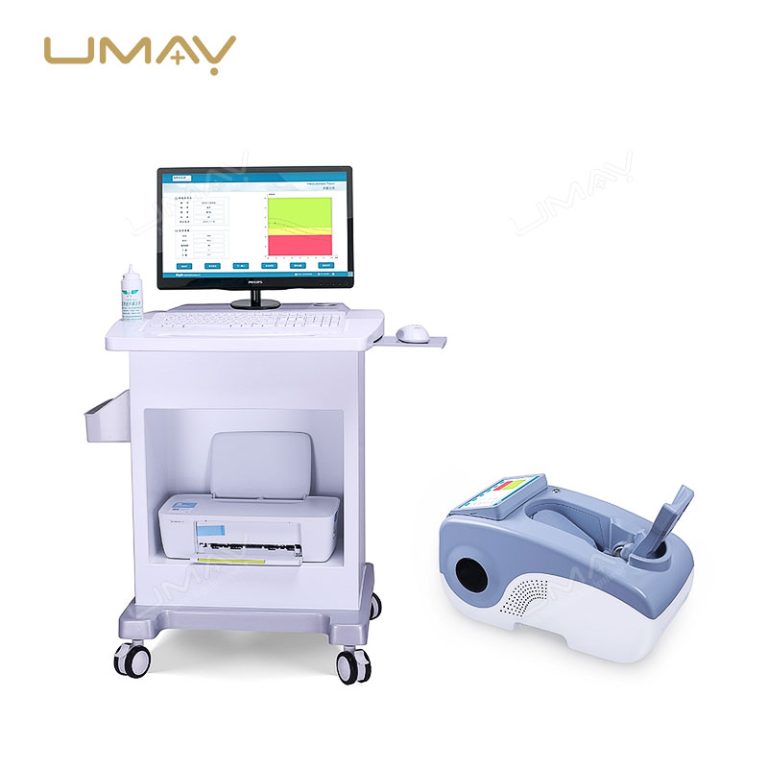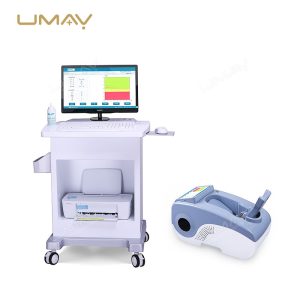

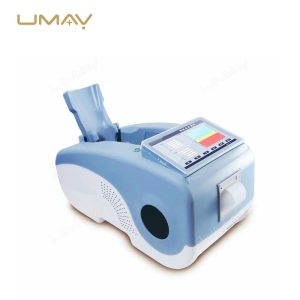




Ultrasound Bone Densitometer with Built-in Computer and Tablet Interface
SKU: UMY-US-048
Brand: Umy
MOQ: 1
Origin: China
Quick Info.
- SKU NO.: UMY-US-048
- Device Classification: Class Ⅱ
- Warranty: 1 Year
- Power Source: Electric
- Transport Package: Carton
- Origin: China
- Material: Metal, Plastic
- After-Sale Service: Online Technical Support
- Production Capacity: 3000 Sets/Year
This ultrasound bone densitometer features an integrated computer and tablet interface, providing efficient, high-resolution bone density assessments for osteoporosis screening and bone health monitoring. Its advanced ultrasound technology delivers accurate, radiation-free measurements, making it safe for regular use in clinics and hospitals. The built-in computer streamlines data processing and storage, enhancing workflow and patient record management. This densitometer is essential for healthcare providers focused on comprehensive and accessible bone health diagnostics.
The Specific Parameters
| ITEMS | PARAMETERS |
|---|---|
| Ultrasound Specifications | |
| Ultrasonic Parameters | BUA (ultrasonic amplitude attenuation), SOS (ultrasonic sound velocity), OI (bone mass index) |
| Probe Frequency | 0.5MHz ± 10% |
| Broadband at 4–6dB | >60% |
| Measurement Method | Full dry type (no liquid inside or outside the equipment, no temperature control required), two-way ultrasonic transmission and reception |
| Measurement Time | ≤ 25 seconds |
| Test Repeatability | OPR ≤ ±1% |
| Measurement Accuracy | SOS ≤ ±2% |
| Test Repeatability (BUA) | ≤ ±5% |
| Diagnostic Parameters | |
| Adult | BUA, OI value, T value, Z value, SOS, OPR, adult ratio, age ratio, obesity index, height prediction, body mass index |
| Children | Z value, BMI, height prediction, obesity, SOS, BUA |
| Output Specifications | |
| Ultrasonic Output (TIS) | 2.8×10^-3 mW/cm² |
| Calibration | Automatic calibration of human body simulation module |
| Temperature Compensation | Automatically adjusts for measurement deviation caused by temperature |
| Reference Databases | Asian, European, Middle Eastern, African |
| Storage and Output | |
| Storage | Supports internal and external printer output reports |
| Report Formats | Diagnostic report output supports PACS network system; A4, B5 formats |
| Interface Output | Dual USB, thermal printer, external printer support |
| Bluetooth and WeChat | Supports Bluetooth report transmission and WeChat code self-download |
| Operational Features | |
| Built-in Screen | 10.1-inch touch screen display |
| Bone Density Software | Includes child and adult test systems with automatic test site prompts |
| Animation Playback | Attracts children’s attention for easier examinations |
| Probe | Oil bladder probe with permanent usability |
| Environmental Requirements | |
| Operating Humidity | 30–70% (non-condensing) |
| Operating Temperature | 10–40°C |
| Power Requirements | AC220V ± 10%, 50Hz, 3.15A, 125W |
| Physical Specifications | |
| Instrument Weight | 13kg |
| Instrument Dimensions | 330mm × 360mm × 645mm (W×H×L) |
| Additional Features | |
| Language Options | Chinese/English interface with golden interface style |
| Operating System Compatibility | Windows XP, 7, 8, 10 (32-bit/64-bit) |
| Case Database | Automatic recording, query, classification, backup; export results to Excel |
| Internet Functions | Supports hospital network systems and remote expert consultations |